Sodium And Chlorine Cation Charge . The reaction between sodium and chlorine. You can use this table to predict whether an atom. It is in group 7 of the periodic table. An electron from each atom feels the attraction from the other. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. A sodium and chlorine atom are near each other. Chlorine has 7 electrons in its outer shell. 93 rows this table shows the most common charges for atoms of the chemical elements. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. the sodium atom is donating its 1 valence electron to the chlorine atom. Ionic bond in sodium chloride. This creates a sodium cation and a.
from oerpub.github.io
93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. Ionic bond in sodium chloride. A sodium and chlorine atom are near each other. An electron from each atom feels the attraction from the other. This creates a sodium cation and a. Chlorine has 7 electrons in its outer shell. It is in group 7 of the periodic table. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,.
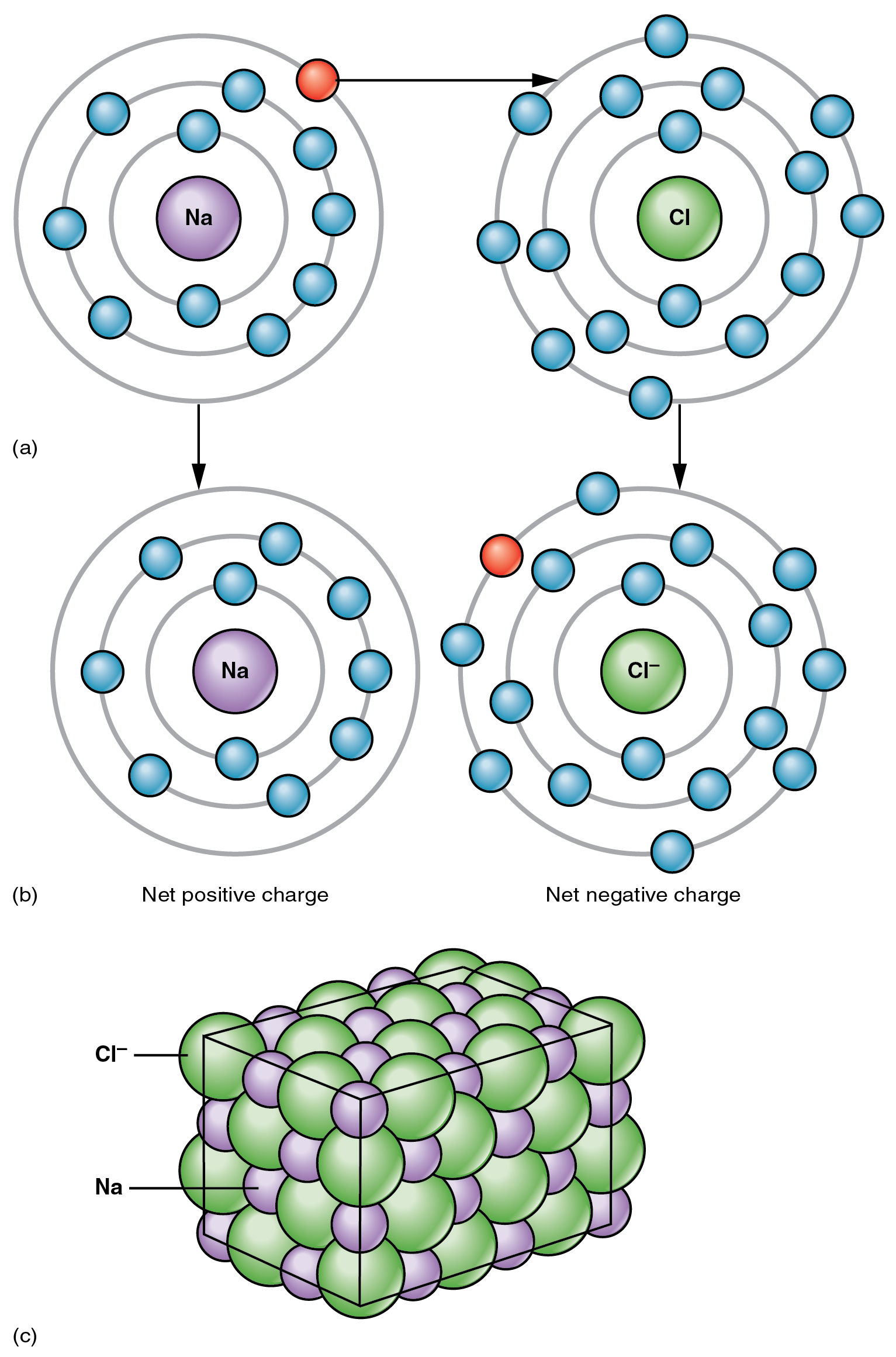
The top panel of this figure shows the orbit model of a sodium atom and
Sodium And Chlorine Cation Charge the sodium atom is donating its 1 valence electron to the chlorine atom. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table to predict whether an atom. An electron from each atom feels the attraction from the other. A sodium and chlorine atom are near each other. This creates a sodium cation and a. It is in group 7 of the periodic table. Chlorine has 7 electrons in its outer shell. the sodium atom is donating its 1 valence electron to the chlorine atom. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. The reaction between sodium and chlorine. Ionic bond in sodium chloride.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Sodium And Chlorine Cation Charge It is in group 7 of the periodic table. You can use this table to predict whether an atom. Ionic bond in sodium chloride. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. 93 rows this table shows the most common charges for atoms of the chemical elements. by losing. Sodium And Chlorine Cation Charge.
From saylordotorg.github.io
Naming Ionic Compounds Sodium And Chlorine Cation Charge A sodium and chlorine atom are near each other. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. This creates a sodium cation and a. An electron from each atom feels the attraction from the other. 93 rows this table shows the. Sodium And Chlorine Cation Charge.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Sodium And Chlorine Cation Charge You can use this table to predict whether an atom. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. The reaction between sodium and chlorine. the sodium atom is donating its 1 valence electron to the chlorine atom. It is in group. Sodium And Chlorine Cation Charge.
From www.numerade.com
SOLVED Question I Fill in the table and write the chemical formula Sodium And Chlorine Cation Charge the sodium atom is donating its 1 valence electron to the chlorine atom. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. You can use this table to predict whether an atom. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the. Sodium And Chlorine Cation Charge.
From www.toppr.com
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos Sodium And Chlorine Cation Charge It is in group 7 of the periodic table. 93 rows this table shows the most common charges for atoms of the chemical elements. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Ionic bond in sodium chloride. A sodium and chlorine atom are near each other. An electron from each. Sodium And Chlorine Cation Charge.
From www.sciencenewsforstudents.org
Explainer Ions and radicals in our world Science News for Students Sodium And Chlorine Cation Charge A sodium and chlorine atom are near each other. This creates a sodium cation and a. It is in group 7 of the periodic table. the sodium atom is donating its 1 valence electron to the chlorine atom. 93 rows this table shows the most common charges for atoms of the chemical elements. You can use this table. Sodium And Chlorine Cation Charge.
From wcponline.com
The Chemistry of Ion Exchange WCP Online Sodium And Chlorine Cation Charge A sodium and chlorine atom are near each other. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. the sodium atom is donating its 1 valence electron to the chlorine atom. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s. Sodium And Chlorine Cation Charge.
From www.toppr.com
the 999] 28. (a 10 (d) 32 uc (c) 32 uc A force Facts between sodium Sodium And Chlorine Cation Charge The reaction between sodium and chlorine. You can use this table to predict whether an atom. An electron from each atom feels the attraction from the other. Ionic bond in sodium chloride. A sodium and chlorine atom are near each other. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. the. Sodium And Chlorine Cation Charge.
From www.flinnsci.ca
Ion Names, Formulas, and Charges Charts for Chemistry Sodium And Chlorine Cation Charge Chlorine has 7 electrons in its outer shell. An electron from each atom feels the attraction from the other. It is in group 7 of the periodic table. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. Ionic bond in sodium chloride. You can use this table to predict whether an atom.. Sodium And Chlorine Cation Charge.
From mavink.com
Sodium Chloride Dot And Cross Diagram Sodium And Chlorine Cation Charge It is in group 7 of the periodic table. An electron from each atom feels the attraction from the other. You can use this table to predict whether an atom. The reaction between sodium and chlorine. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. by losing an electron to become. Sodium And Chlorine Cation Charge.
From drmchemistrytutor.com
sodium chloride ions Dr. M. Chemistry Tutor Sodium And Chlorine Cation Charge Chlorine has 7 electrons in its outer shell. This creates a sodium cation and a. You can use this table to predict whether an atom. It is in group 7 of the periodic table. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. A sodium and chlorine atom are near each other.. Sodium And Chlorine Cation Charge.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Sodium And Chlorine Cation Charge the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. A sodium and chlorine atom are near each other. The reaction between sodium and chlorine. An electron. Sodium And Chlorine Cation Charge.
From www.numerade.com
SOLVED Table 4 Ionic Compounds and their Names Formula of Cation and Sodium And Chlorine Cation Charge It is in group 7 of the periodic table. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. Ionic bond in sodium chloride. An electron from each atom feels the attraction from the other. A sodium and chlorine atom are near each other.. Sodium And Chlorine Cation Charge.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Sodium And Chlorine Cation Charge It is in group 7 of the periodic table. A sodium and chlorine atom are near each other. Ionic bond in sodium chloride. An electron from each atom feels the attraction from the other. Chlorine has 7 electrons in its outer shell. You can use this table to predict whether an atom. the sodium atom is donating its 1. Sodium And Chlorine Cation Charge.
From manuallistgelsemine.z13.web.core.windows.net
Dot Cross Diagram For Sodium Chloride Sodium And Chlorine Cation Charge This creates a sodium cation and a. by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. 93 rows this table shows the most common charges for atoms of the chemical elements. Ionic bond in sodium chloride. It is in group 7 of. Sodium And Chlorine Cation Charge.
From schematicsarjairllixq7.z22.web.core.windows.net
Chlorine Electric Dot Diagram Sodium And Chlorine Cation Charge The reaction between sodium and chlorine. An electron from each atom feels the attraction from the other. A sodium and chlorine atom are near each other. 93 rows this table shows the most common charges for atoms of the chemical elements. This creates a sodium cation and a. Ionic bond in sodium chloride. It is in group 7 of. Sodium And Chlorine Cation Charge.
From fr.dreamstime.com
Anions Et Cations Atomes Par Exemple De Sodium Et De Chlore Sodium And Chlorine Cation Charge by losing an electron to become the na + cation, sodium’s underlying shell of 8 electrons becomes the ion’s outer shell with a stable octet. Ionic bond in sodium chloride. the sodium atom is donating its 1 valence electron to the chlorine atom. A sodium and chlorine atom are near each other. An electron from each atom feels. Sodium And Chlorine Cation Charge.
From www.out-class.org
Difference Between Cations and Anions OutClass Sodium And Chlorine Cation Charge An electron from each atom feels the attraction from the other. It is in group 7 of the periodic table. Ionic bond in sodium chloride. A sodium and chlorine atom are near each other. This creates a sodium cation and a. the vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride,. 93. Sodium And Chlorine Cation Charge.